Cosmetic Injectables in Australia: Our Commitment to Safe and Compliant Care
By Radhika Narayan-Nath

At Plaza Central Medical & Aesthetics, we believe cosmetic treatments should always be performed safely, ethically, and transparently. As a doctor-led clinic in Maroochydore, all cosmetic services — including cosmetic injectables — are conducted under strict clinical supervision and within AHPRA and TGA guidelines.
Recent national changes have highlighted the importance of maintaining compliance when advertising and providing these treatments. Here’s how we ensure our patients receive safe care and clear, responsible information.
What Are Cosmetic Injectables?
Cosmetic injectables are prescription-only medicines (Schedule 4 drugs) used to address facial lines, wrinkles, and volume loss. Because these are prescription products, Australian law prohibits clinics from directly naming, displaying, or promoting them publicly. Instead, treatment suitability can only be discussed during a private medical consultation with an authorised prescriber.
At Plaza Central, every treatment plan begins with a comprehensive medical assessment to determine the most appropriate approach for each individual.
Staying Compliant: AHPRA & TGA Rules We Follow
No Product Advertising
We never name or indirectly refer to prescription-only medicines (for example, brand names or abbreviations). Our communication focuses on consultations, safety, and patient education — not on product promotion or price comparison.
Clinician-Led Consultations
All procedures are performed by a registered nurse cosmetic practitioner, in collaboration with our supervising general practitioners. A qualified prescriber assesses each patient before any treatment.
Informed Consent
Before every procedure, we provide written and verbal consent forms covering: risks and benefits, expected outcomes and recovery, practitioner qualifications, costs and follow-up recommendations. This ensures patients are fully informed and comfortable before proceeding.
Transparent, Educational Communication
We use our platforms — such as blogs, newsletters, and social media — for education and awareness, not advertising. Posts about skin health, ageing, and consultation benefits help patients make informed decisions, without breaching promotional rules for Schedule 4 products.
Responsible Imagery and Messaging
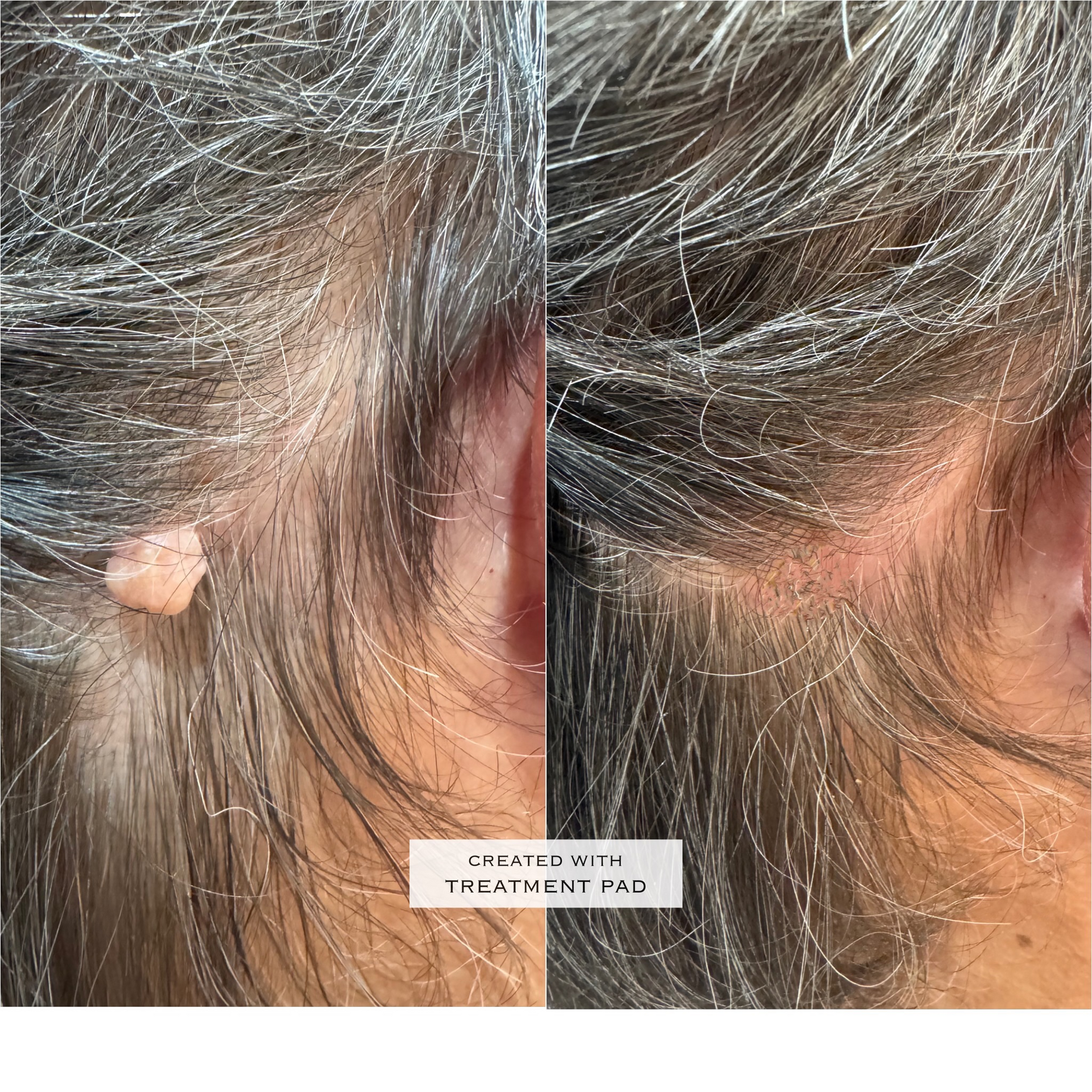
We do not use edited or exaggerated “before and after” images. Our visuals are professional, factual, and never target minors or imply unrealistic results.
Review and Moderation
Any feedback or social media comment that could be interpreted as a testimonial about clinical care is removed or hidden in line with AHPRA requirements.
Why These Rules Matter
These national regulations protect both patients and practitioners. The Therapeutic Goods Administration (TGA) restricts the advertising of prescription medicines, while the Australian Health Practitioner Regulation Agency (AHPRA) ensures that all health advertising is factual, evidence-based, and ethical.
By following these standards, we maintain transparency in what treatments involve, trust between our clinic and our community, and safety through clinician-led decision-making.
Education, not promotion
At Plaza Central Medical & Aesthetics, we focus on patient education — helping you understand how non-surgical treatments can complement healthy ageing, the importance of practitioner training and product safety, and why consultation-based care ensures the best and safest outcomes.
We’re proud to lead by example, showing that cosmetic medicine can be both beautiful and responsible.
Booking a Consultation
If you’re considering aesthetic treatments, we invite you to book a confidential consultation with our team. During this appointment, we’ll discuss your goals, assess your suitability, outline all available options, and explain any potential risks or downtime.
Our Location:
Plaza Central Medical & Aesthetics
10–18 Pikki Street, Maroochydore QLD
Phone:
(07) 5343 7660
Website: plazacentralmedical.com.au
References
· AHPRA: Advertising a Regulated Health Service – Guidelines under Section 133 of the National Law (2024 update)
· TGA: Advertising Health Services & Cosmetic Injections – Guidance for Practitioners (Version 2.0, May 2024)
Read our blogs